There are several parameters which affect dispersion and solubility of cmc in water. These parameters include: solvent, polymer (viscosity and degree of substitution) and the amount of shear rate. In this article we are going to study some important parameters and their effects on the performance of cmc.
Solvent
Cmc is soluble in cold and hot water and it is insoluble in organic and nonpolar solvents. However, this substance soluble in mixture of water and water-soluble solvents like alcohol and acetone. Solutions with low concentration of cmc can be prepared in the presence of 50 weight percent of alcohol or 40 weight percent of acetone. However, sometimes aqueous solutions of cmc can tolerate even higher concentration of alcohol and acetone in the solution. In general, it is obvious those grades of CMC which have lower viscosity, can tolerate higher concentration of alchohal and acetone in their solutions.
Chemical structure of CMC
As the degree of substitution increases and the molecular weight decreases, the rate of solubility of CMC increases. CMC particle size has significant effect on dispersion and solubility properties. For instance, experimentally it has been found that when the turbulence caused by mixer is not enough and vortex cannot be formed in the solution, coarse and big granules have better and easier dispersion compared to soft and small granules. However, it is worth mentioning that when the size of granules become bigger, the time needed to solve or disperse them in the solution also rises. Hence, in the cases which high rate of solubility is required, the best option is utilizing the CMC with small and soft particle size. However, in this condition in order to reach an appropriate dispersion, we need to use special techniques like: Pre-wetting CMC powder by another liquid ( liquids which do not cause swelling), mixing CMC with other dry materials or utilizing [1] Eductor-type mixing device.
Appropriate dispersing methods
When CMC particles are added to water, they tend to aggregate and form lumps. In order to have easy and good dispersion, dissolving process should include a two-step process.
- Suitable dispersion of dry powder in the water: Particles should get wet separately and we should not have any aggregation in the system.
- Wet particle should dissolve in the media
When we use an appropriate method, dispersion occurs very well and CMC dissolves so rapidly. In order to have a transparent solution which has no undissolved lump, several methods can be used.
Method 1
Add CMC to the vortex of vigorously agitated water. The rate of addition must be slow enough to permit the particles to separate and their surfaces to become individually wetted, but it should be fast enough to minimize viscosity buildup of the aqueous phase while the CMC is being added.
Method 2
Prior to addition of CMC to water, wet the powder with a water-miscible liquid such as alcohol, glycol, or glycerol that will not cause CMC to swell. Two to three parts of liquid per part of CMC should be sufficient.
Method 3
Dry-blend the CMC with any dry, non-polymeric material used in the formulation. Preferably, the CMC should be less than 20% of the total blend.
Method 4
Use a water eductor (Figure 1) to wet out the polymer particles rapidly. The polymer is fed into a water-jet eductor, where a high-velocity water-flow instantly wets out each particle, thus preventing lumping. This procedure speeds solution preparation and is particularly useful where large volumes of solutions are required. For users wishing the convenience of an automatic system, a polymer solution preparation system (PSP), which is used in conjunction with a water eductor, is can be suggested. This system is shown in Figure 2.
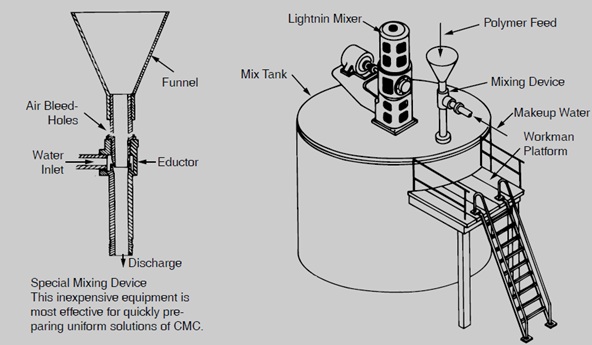
Figure1) Typical Installation of Eductor-Type Mixing Device
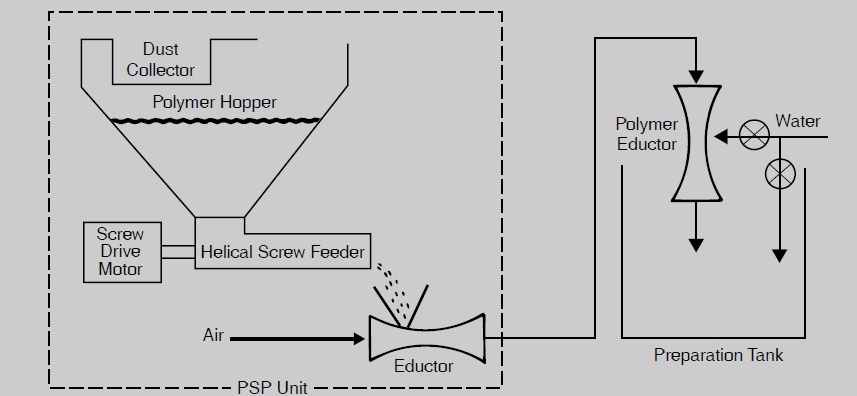
Figure2) Automated Polymer Solution Preparation (PSP) System